Familiarity and Perceptions of Ovarian Cancer Biomarker Testing and Targeted Therapy: A Survey of Oncology Nurses in the United States
Objectives: To assess oncology nurses’ awareness of biomarker testing and targeted therapy for ovarian cancer.
Sample & Setting: 100 oncology nurses completed an online survey in June 2022.
Methods & Variables: A cross-sectional survey was used to examine nurses’ understanding of ovarian cancer testing and treatments, assess barriers, and identify opportunities for further education.
Results: Almost all respondents believed biomarker testing and targeted therapy were very/extremely important in diagnosing and supporting treatment of patients with ovarian cancer. Nurses were very/extremely familiar with cancer antigen 125 and germline testing, but fewer reported the same familiarity with somatic testing. Most nurses were familiar with targeted therapy for ovarian cancer, but only about half were very/extremely familiar with poly(ADP-ribose) polymerase (PARP) inhibitors. Less than half felt highly knowledgeable about PARP inhibitors.
Implications for Nursing: It is important that oncology nurses understand biomarker testing and targeted therapy. There is an opportunity to provide resources to nurses to help them become more comfortable with PARP inhibitors in particular.
Jump to a section
Oncology care has shifted in the past 30 years from broad chemotherapeutics, which selectively remove rapidly growing/cycling cells, to targeted therapies that inhibit signaling pathways specifically activated within that cancer type (Falzone et al., 2018; Hanes, 2020; Miller, 2022; Shahid et al., 2019). Precision medicine is defined as “a form of medicine that uses information about a person’s own genes or proteins to prevent, diagnose, or treat disease. In cancer, precision medicine uses specific information about a person’s tumor to help make a diagnosis, plan treatment, find out how well treatment is working, or make a prognosis” (National Cancer Institute, n.d.). Specifically, precision oncology uses biomarkers—a variety of molecular, genetic, and genomic data—to identify patients who are most likely to respond to a particular treatment (Senft et al., 2017). Precision oncology may improve cancer care by increasing survival and improving patients’ quality of life, but it is complex and continually evolving (Lassen et al., 2021; Mateo et al., 2022).
Although targeted therapies have been widely used in other cancers, such as breast, lung, and colon, a literature review conducted for the purpose of this study found that they are relatively new to the management of ovarian cancer (Diab & Muallem, 2017; Guan & Lu, 2018; Lim & Ledger, 2016; Wang et al., 2020). In addition, the lower incidence of ovarian cancer compared with other cancers (American Cancer Society, 2023a, 2023b, 2023c) means that oncology nurses may encounter this diagnosis less often in practice. Ovarian cancer care is often provided in a subspecialized setting, such as a gynecologic oncology practice; therefore, nurses in general medical oncology practices have smaller volumes of patients with ovarian cancer.
Treatment options for ovarian cancer (epithelial, fallopian tube, primary peritoneal) have been limited to date, with therapies historically constrained to surgery and chemotherapy (Guan & Lu, 2018), but they are evolving to include a small number of targeted therapies with distinct indications for first-line, maintenance, and/or recurrent settings (Abdelmeseh et al., 2021; Konstantinopoulos, Lheureux, & Moore, 2020; Nag et al., 2022; Nero et al., 2021). Targeted therapy includes angiogenesis inhibitors, which block creation of new blood vessels and inhibit tumor growth (Hall et al., 2013); poly(ADP-ribose) polymerase (PARP) inhibitors, which prevent cancer cells from repairing the damage in their DNA, leading to apoptosis (Evans & Matulonis, 2017; O’Malley et al., 2022; Slade, 2020); and tumor-agnostic tyrosine receptor kinase (TRK) inhibitors, which block the TRK protein that controls cell proliferation and metabolism (Basu et al., 2018; Hall et al., 2013; Jiang et al., 2021). Because TRK biomarkers are rarely found in ovarian cancer, TRK inhibitors are infrequently used for treating it (Endo et al., 2022). Patients who experience recurrence of their disease or develop resistance to platinum-based chemotherapy may be eligible for other tumor-agnostic targeted therapy or immunotherapy based on genomic features of their cancer (Abdelmeseh et al., 2021; Kandalaft et al., 2020; Nero et al., 2021).
Biomarker testing can help determine the likelihood of benefitting from targeted therapy. Cancer antigen 125 (CA-125) testing is a biomarker that has been used historically in the setting of ovarian cancer but as a means to assess prognosis, to estimate risk, and as a proxy to indicate recurrence/progression of disease (Gadducci et al., 2004; Juretzka et al., 2007; National Comprehensive Cancer Network [NCCN], 2022a; Santillan et al., 2005). Cancers caused by hereditary pathogenic germline variants account for about 5%–10% of all cancers (Anand et al., 2008; Tsaousis et al., 2019). Germline (i.e., genetic) testing can be performed on blood, saliva, or cultured fibroblasts to determine whether there is an underlying hereditary cancer predisposition. However, most cancers are caused by somatic, or acquired, genomic variants. Somatic (tumor) biomarkers are identified using blood or tumor tissue and can aid in determining diagnosis, prognosis, and predictive benefit of targeted therapeutic options (NCCN, 2022b). However, the analysis and interpretation of genomic testing results can be complex and may not lead to the identification of an actionable variant with an approved targeted treatment (Chakravarty et al., 2022). The NCCN guidelines recommend that all patients with ovarian cancer undergo genetic risk evaluation and germline and somatic testing as part of a comprehensive diagnostic workup. In partnership with several oncologic societies and patient advocacy groups, an interprofessional expert steering committee offers recommendations to improve the quality of ovarian cancer care, including biomarker testing for all patients (Temkin et al., 2022).
Oncology nurses are uniquely positioned to facilitate patient education and understanding of the implications of precision oncology (Nevidjon, 2018), but the rapid advancement of targeted therapies presents challenges in managing patient care. A virtual focus group conducted by the Oncology Nursing Society (ONS, 2021) among 17 oncology nurses found a lack of familiarity and comfort with precision oncology. As PARP inhibitors demonstrate an improvement in progression-free survival in patients with ovarian cancer, it is important that oncology nurses understand the role that these and other targeted therapies play in the treatment of ovarian cancer, so they can inform, guide, and support their patients (Hollasch, 2022; Pothuri et al., 2020).
To better understand the nursing role in precision therapy for ovarian cancer, the authors conducted a study to assess oncology nurses’ awareness of biomarker testing, measure knowledge about various treatments for ovarian cancer, determine barriers to diagnostic testing and treatments, and identify opportunities to provide further education and resources.
Methods
Sample and Setting
Oncology nurses from an ONS-provided list and online panel companies were invited to participate in the survey between June 8, and June 28, 2022. Potential respondents qualified for the survey if they practiced in the United States, had been in oncology nursing practice for at least two years, spent at least 70% of their time in direct patient care, treated at least 10 patients with ovarian cancer per year, and were familiar with targeted therapies in general and PARP inhibitors in particular. Because respondents had to be somewhat familiar with specific ovarian cancer treatment types, a sample size of 100 was deemed sufficient for generalizing to the population of oncology nurses in the United States.
Design and Data Collection
This was a cross-sectional online survey of oncology nurses who were recruited via email through an online panel company with which respondents had provided permission to be contacted for research purposes. The list of nurses from ONS was matched to the online panel; nurses who were also members of the online panel were invited to take the survey. The email invitation described the nature of the study; only respondents who consented to participate were allowed to continue onto the screening portion of the survey. Survey data were collected using Decipher online survey software.
Reminder emails were sent to nonrespondents. Participation in the survey was voluntary, and respondents could discontinue taking the survey at any time. No personally identifying information was included in the dataset; data were analyzed in aggregate. The study protocol was submitted to WCG Institutional Review Board for ethical approval, which determined that the research qualified for exemption status.
Measures
The survey consisted of a variety of numeric-text, open-text, and multiple-choice questions, and five-point Likert-type scales to assess familiarity, importance, comfort, knowledge level, and the degree to which nurses find tasks to be challenging. Survey questions focused on ovarian cancer treatments and testing, as well as the responsibilities and challenges faced by oncology nurses. Questions regarding clinical practice and demographics were also included in the survey. Pretest interviews were conducted with three oncology nurses to assess the face validity of the survey. Minor modifications were made to the survey for clarity and comprehension.
Data Analysis
Descriptive statistical analyses (means and frequencies) were performed using Q Research Software for Windows 23. Data are presented as number and percentage for categorical variables, and continuous data are expressed as mean and SD unless otherwise specified. Percentile values are rounded to the nearest whole number. Open-text (unaided) questions were coded and reported as categories of responses.
Results
Sample Characteristics
Of the 459 respondents who entered the survey, 100 oncology nurses completed the survey (22%); 280 did not meet the qualification criteria, and the remaining respondents did not finish the survey. The median survey length was 19 minutes. The majority of respondents were nurse practitioners or staff nurses, with a mean of 18 years in practice. The nurses reported treating an average of 48.8 patients with ovarian cancer in the past year. Most reported practicing in academic/university hospitals or outpatient centers; 43% practiced in an outpatient setting. Characteristics of survey respondents are described in Table 1. The oncology nurses surveyed reported that among the patients they treated with ovarian cancer within the past year, 40% were diagnosed with epithelial ovarian cancer, 14% with germ cell ovarian cancer, and 10% with stromal cell ovarian cancer; 37% of nurses were not sure of the specific proportions of patients with each ovarian cancer type.
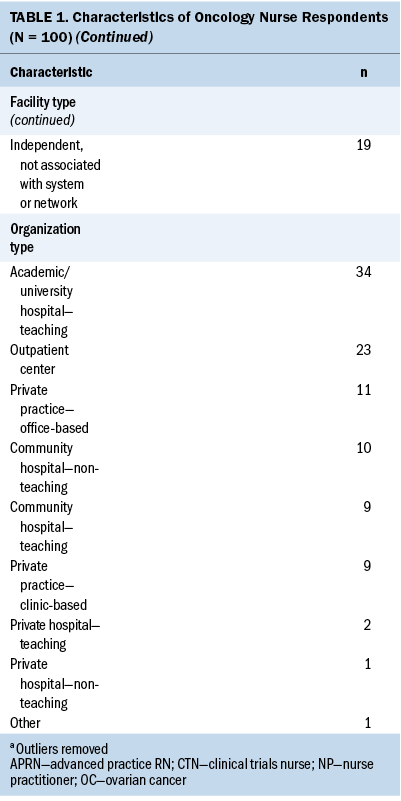
Ovarian Cancer Testing
When asked to list the types of diagnostic and treatment testing for ovarian cancer (unaided) they were aware of, oncology nurses were most likely to report being aware of imaging tests including ultrasound, computed tomography, magnetic resonance imaging, positron-emission tomography (82%), and blood tests including CA-125 (68%). Less than half were aware of genetic testing including molecular testing and next-generation sequencing (45%), and tumor testing including homologous recombination deficiency testing and tumor markers (18%). When presented with a list of tests, the majority of nurses in the survey reported being very familiar with blood tests (94%), imaging (90%), biopsy (82%), and surgery (73%) for diagnosing ovarian cancer. Fewer nurses were similarly familiar with biomarker testing (60%); 34% were somewhat familiar and 6% were not at all/not very familiar with them.
Oncology nurses reported varying degrees of familiarity with specific tests associated with ovarian cancer biomarker testing. CA-125 and germline testing were very/extremely familiar to the majority of nurses participating in the survey (see Figure 1). Only 38% of oncology nurses reported being equally familiar with somatic testing; more than one-fourth were not at all/not very familiar with this type of biomarker testing. Among oncology nurses at least somewhat familiar with each test for ovarian cancer, most felt very/extremely knowledgeable about blood tests (81%), imaging (73%), and biopsy (66%), but only about half (51%) had this degree of knowledge about biomarker testing.
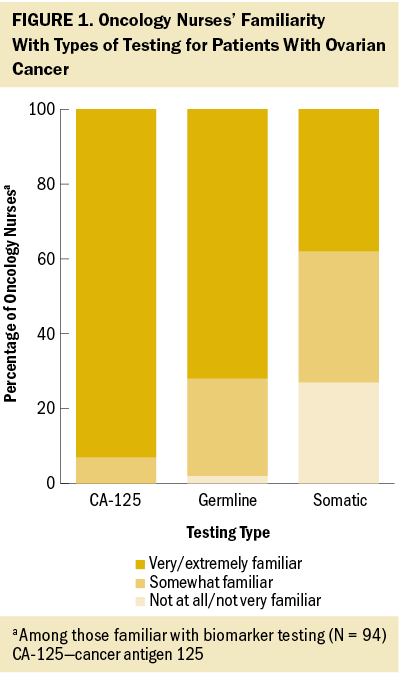
Comfort with explaining the rationale for biomarker testing with their patients also varied. Most oncology nurses were very/extremely comfortable explaining CA-125 testing (see Figure 2). Fewer nurses were as comfortable explaining germline testing and somatic testing to their patients. Almost all oncology nurses surveyed felt biomarker testing for ovarian cancer was very/extremely important in diagnosing and supporting the treatment of patients with ovarian cancer (91%), similar to the level of importance of biopsies (98%) and imaging (97%). However, nurses reported fewer patients treated in the past year receiving biomarker testing (75%) as compared to imaging (92%) and biopsies (85%).
Most oncology nurses familiar with CA-125 testing reported that CA-125 was not at all/not very challenging to coordinate or arrange (80%) for their patients with ovarian cancer but felt that germline and somatic testing were at least somewhat challenging (64% and 79%, respectively). When asked to describe the main barriers or challenges with biomarker testing for patients with ovarian cancer in an unaided manner, oncology nurses most commonly mentioned getting insurance approvals (35%) and financial barriers (26%); only 21% reported that there were no barriers.
Ovarian Cancer Treatment
Unaided, the oncology nurses in the authors’ survey were most commonly aware of chemotherapy (95%) and surgery (69%) for the treatment of ovarian cancer. About one-third listed PARP inhibitors (34%) and targeted therapy without specifying a type of treatment (29%). Oncology nurses reported being very/extremely familiar with chemotherapy (96%), immunotherapy (84%), surgery (79%), targeted therapy (78%), hormone therapy (77%), and radiation therapy (51%). Among nurses familiar with targeted therapy, familiarity with specific types of targeted therapy varied widely, with most being very/extremely familiar with angiogenesis inhibitors and few familiar with TRK inhibitors (see Figure 3). Among those at least slightly familiar with each ovarian cancer treatment type, oncology nurses reported being very/extremely knowledgeable about chemotherapy (86%), immunotherapy (76%), hormone therapy (65%), targeted therapy (58%), surgery (55%), and radiation therapy (39%).
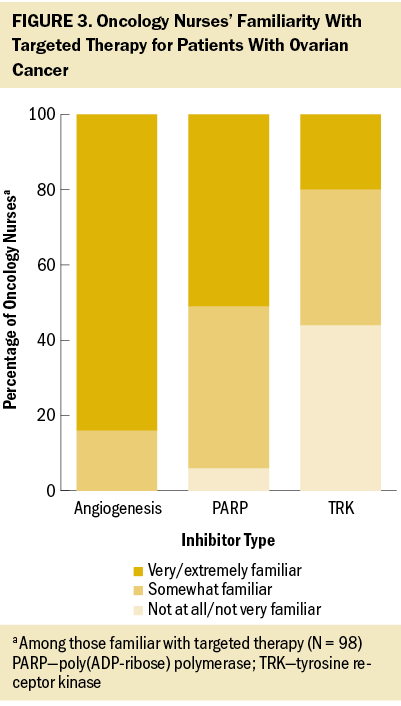
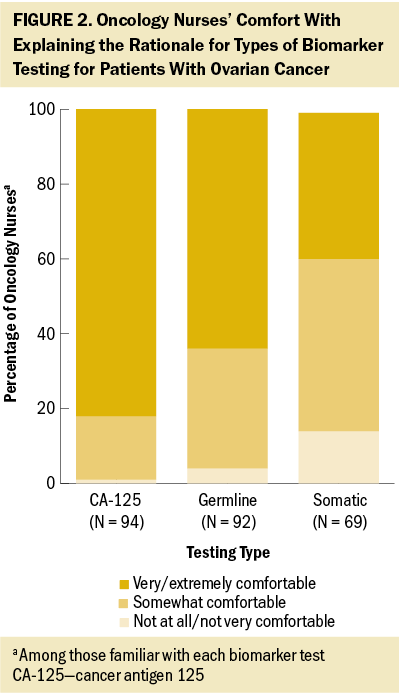
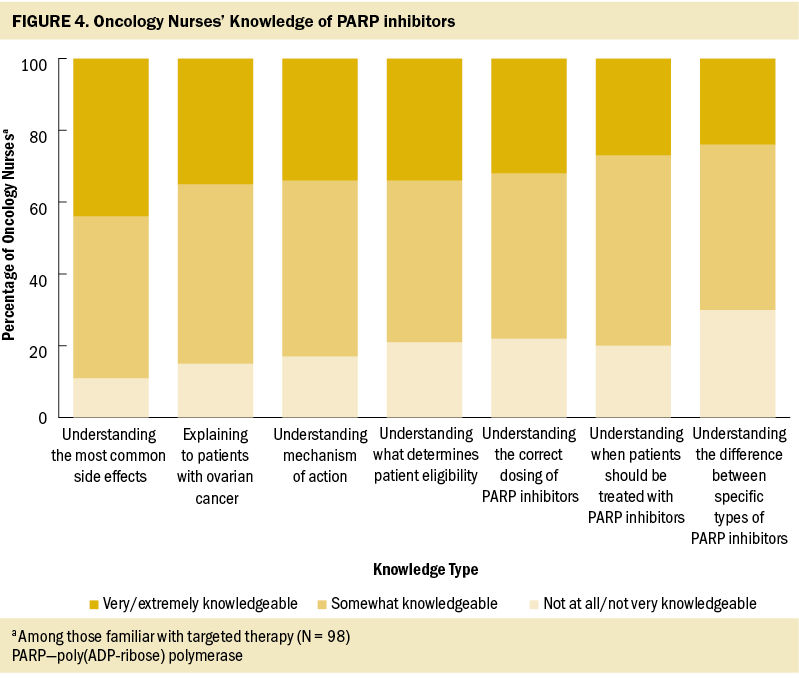
Targeted therapy was considered very/extremely important in treating patients with ovarian cancer according to nearly all nurses in the authors’ survey (93%), similar to chemotherapy (99%), surgery (94%), and immunotherapy (86%). However, nurses reported that less than half (47%) of their patients with ovarian cancer treated in the past year had received targeted therapy, similar to the proportion of patients receiving immunotherapy (46%) and hormone therapy (38%). Among the oncology nurses the authors surveyed who were familiar with angiogenesis inhibitors, most were very/extremely comfortable explaining the mechanism of action (78%) and side effects (80%) to their patients. Fewer nurses expressed this same level of comfort with explaining the process and side effects of PARP inhibitors (45% and 59%, respectively) and of TRK inhibitors (40% and 51%, respectively) among those familiar with each treatment type. Less than half of the oncology nurses surveyed reported feeling very/extremely knowledgeable about specific aspects of PARP inhibitors; almost one-third believed they had little or no understanding of the differences between various PARP agents (see Figure 4).
Ovarian Cancer Management
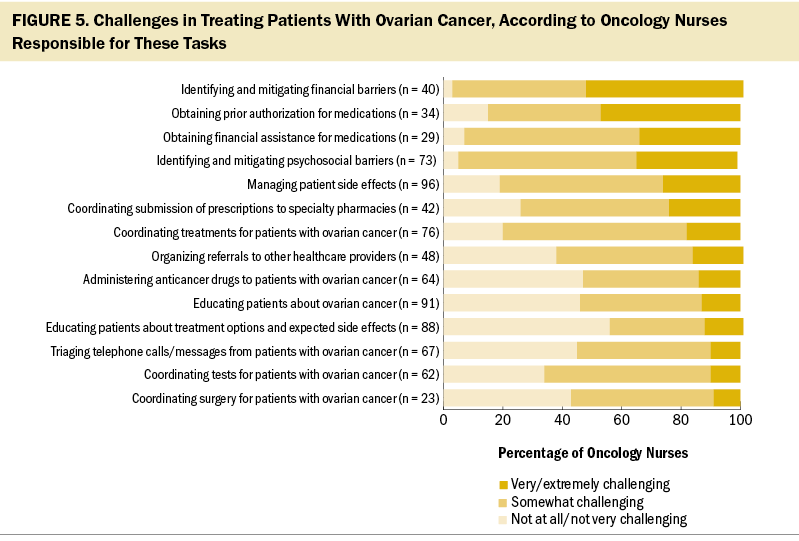
Most nurses reported being responsible for managing patient side effects (96%), educating patients about ovarian cancer (91%) or treatment options and side effects (88%), coordinating treatments for patients (76%), identifying and mitigating psychosocial barriers to treatment (73%), triaging telephone calls/messages from patients (67%), administering anticancer drugs (64%), and coordinating tests for their patients with ovarian cancer (62%). Fewer reported being responsible for identifying and mitigating financial barriers to treatment (40%), obtaining prior authorization for medications (34%), and obtaining financial assistance for treatments (29%).
When asked to describe (unaided) the top three challenges of treating and managing their patients with ovarian cancer, oncology nurses were most likely to cite managing side effects (51%), financial barriers (45%), and emotional/psychosocial issues (31%). Oncology nurses were then presented with the list of tasks they were responsible for and asked to indicate how challenging they found each; managing the financial aspects of treatment was considered the most challenging, followed by aspects of patient care (i.e., addressing psychosocial barriers and managing side effects) (see Figure 5).
Discussion
Treatments for ovarian cancer have quickly evolved to include targeted therapies. To best care for and support patients in their treatment journey, oncology nurses must have current information to guide their understanding and use of biomarker testing and targeted treatments in ovarian cancer. This study sought to assess oncology nurses’ familiarity with and understanding of targeted therapies in general, and PARP inhibitors in particular. To the authors’ knowledge, this is the first quantitative evaluation of oncology nurses’ knowledge about biomarker testing and targeted therapies for ovarian cancer.
In the authors’ quantitative survey, they found that the most challenging aspects regarding ovarian cancer management among oncology nurses responsible for these tasks were managing side effects and mitigating financial issues and psychosocial barriers, similar to the findings of a focus group of oncology nurses exploring biomarker testing and targeted therapy (ONS, 2022). Almost all the oncology nurses surveyed considered biomarker testing for ovarian cancer to be highly important, almost to the same degree as biopsies and imaging tests. The majority of nurses were highly familiar with biomarker testing in general, but this was limited to CA-125 and germline testing because fewer than 4 in 10 nurses were very familiar with somatic testing. Only half of the oncology nurses felt highly knowledgeable about biomarker testing. Few nurses were very comfortable explaining the rationale for somatic testing with their patients with ovarian cancer, and most considered this testing to be challenging to coordinate or arrange.
Biomarker and genetic testing are important components of effective cancer care (Ruddy et al., 2016; Temkin et al., 2022). Qualitative interviews in a study of patients with ovarian cancer who received genetic testing found that information about genetic testing needed to be relevant and understandable; preferred sources of information about genetic testing varied and included digital and print information as well as discussions with healthcare professionals (Zhang et al., 2022). Oncology nurses participating in a focus group concurred; they stressed the importance of explaining testing in a way patients can understand, necessitating knowledge of key terminology to support communication (ONS, 2022). Similar to what the authors found for biomarker testing, almost all the nurses surveyed believed targeted therapies were very important in the treatment of ovarian cancer and were on par with other treatments, including chemotherapy. Although most oncology nurses were highly familiar with targeted therapies in general, only half were aware of PARP inhibitors. In addition, few nurses indicated a high degree of understanding of the mechanism of action of PARP inhibitors, when patients should be treated with them, and how to determine whether patients are eligible for treatment. Comfort in explaining the process of treatment with PARP inhibitors and their side effects was low among the oncology nurses surveyed, as compared with angiogenesis inhibitors.
In the United States, three PARP inhibitors, olaparib, rucaparib, and niraparib, have been approved for maintenance treatment of ovarian cancer (AstraZeneca, 2020; Clovis Oncology, 2022; GlaxoSmithKline, 2020) and can be valuable additions to the treatment options healthcare professionals have at their disposal (Pothuri et al., 2020). A meta-analysis of PARP inhibitors approved for patients with recurrent ovarian cancer demonstrated their effectiveness in prolonging progression-free survival (Wang et al., 2021). Although these treatments were associated with an increase in adverse events in patients with recurrent ovarian cancer as compared with placebo, the side effects were tolerable (Wang et al., 2021). Changes in the mechanism of action of cancer treatments during the past few decades (Falzone et al., 2018; Hanes, 2020; Miller, 2022; Shahid et al., 2019) could be a key reason for the lack of familiarity with PARP inhibitors among oncology nurses. Cell cycle inhibitors and traditional chemotherapy treatments are well known among oncology nurses, but the burden of having to learn the mechanisms for various cell signaling pathways and how targeted therapies act on those pathways can be challenging.
Oncology nurses and nurse navigators are key members of a patient’s care team, playing an important role in coordinating, guiding, and supporting patients’ treatment goals, plans, and management (Nevidjon, 2018; ONS, n.d.; Reed & Rua, 2020; Temkin et al., 2022), as well as helping to support and mitigate patients’ psychosocial issues (O’Sullivan et al., 2011). The current study shows that as therapies for ovarian cancer quicky evolve, knowledge and expertise have not kept pace. Education and resources are needed to increase awareness, familiarity, and comfort with biomarker testing and targeted therapies among oncology nurses. These results build on and confirm the findings from the virtual focus group conducted by ONS (2021) that revealed a lack of comfort and familiarity regarding precision oncology, including immunology and biomarker testing. The qualitative research demonstrated the difficulty in staying informed in a constantly changing testing and treatment landscape, but having clear and current information is critical for oncology nurses to be more comfortable with precision oncology as they guide their patients’ care (ONS, 2021).
ONS (2021) research identified the need for patient-focused materials for oncology nurses to provide to their patients, and resources and tools for oncology nurses’ own education and knowledge including in-services, web-based self-directed training, brief updates with new information and guidelines, and care management pathways. Guidelines on germline and somatic testing in ovarian cancer care offered by the American Society of Clinical Oncology (Konstantinopoulos, Lacchetti, & Annunziata, 2020) and testing and treatment guidelines provided by NCCN for healthcare professionals (NCCN, 2022a) and patients (NCCN, 2022b) can be useful resources.
Limitations
There are several limitations to the current study. Selection bias could exist because participants were not randomly selected from the population of all oncology nurses. Nurses responding to the survey may be different than nonresponders, which could limit the generalizability of the study findings to all oncology nurses in the United States. Nurses belonging to an online panel could differ demographically from nurses who are not members of a research panel. About two-thirds of the nurses surveyed in the current study had been in oncology nursing practice for at least 15 years; therefore, the findings could differ for nurses newer to the field. A small proportion of the study sample was from the western United States, in rural areas, and private practice or community hospital settings; results may be different for nurses in these clinical practice settings.
Responder bias could also be a limitation, but based on the survey responses indicating a lack of familiarity and comfort on the study topics, respondents were likely truthful in their responses. By not revealing the specific topic of the survey to respondents until they met the required screening criteria, the potential for responder bias was mitigated. The survey focused on specific aspects of biomarker testing and targeted therapies, and thus was not intended to delve into specific knowledge and understanding of all available screening and diagnostic testing and treatments for ovarian cancer. In addition, although face validity was assessed with pretesting, the survey was not formally tested or validated.
Implications for Nursing and Research
It is important for oncology nurses to understand targeted therapy. Seeking out this knowledge will help ensure nurses can competently provide for their patients with ovarian cancer. Oncology nurses need to be comfortable addressing questions from patients about how PARP inhibitors work, how they differ from other therapies they have tried, and the expected side effects. There is a need for education and resources to help nurses gain the understanding of targeted therapies to best support and care for their patients with ovarian cancer. This could be provided via various channels including continued nursing education, self-directed training, certificate programs, education/training offered by the institutions where nurses practice, and nursing organizations such as ONS. However, further research is needed to fully explore the underpinnings of the knowledge gap to determine whether the issue is simply a lack of information, or whether there are institutional or environmental factors that play a role, such as staffing levels, burnout, prioritization by leadership, and the availability of dedicated time for continued education. In addition, a deeper analysis of the education needed by nurses who practice in specific settings (e.g., inpatient versus outpatient, medical oncology versus gynecologic oncology) could aid in developing and targeting educational resources.
It is also critical that nurses understand the financial challenges associated with biomarker testing and oral anticancer therapy (inclusive of, but not specific to, PARP inhibitors) (ONS, 2022). Even if they are not responsible for performing this work, nurses must understand the challenges faced by their patients to anticipate and help address their needs. Oncology nurses should understand who is responsible for handling financial issues in their practices to ensure efficient communication and collaboration, which can improve the patient experience. If their practices do not have designated or adequate staffing to support financial navigation, nurses should be empowered to advocate for this resource.
Where these resources are available, there is also an opportunity to strengthen collaborative partnerships with genetic counselors, nurse navigators, and financial advocates who could help inform and support the educational needs and financial challenges of targeted therapy. Educational materials and resources should be formally assessed to ensure they are delivering accurate and useful information. Evaluating oncology nurses’ familiarity with biomarker testing and targeted therapies after engaging in educational activities can help ensure that resources and tools continually evolve to best support them as they provide care to their patients with ovarian cancer.

Conclusion
Although oncology nurses believe biomarker testing and targeted therapy are important for the diagnosis and treatment of ovarian cancer, they lack familiarity, knowledge, and comfort with specific types of tests and treatments. Nurses could benefit from more education and resources about biomarker testing and targeted therapy in general and PARP inhibitors in particular. Being armed with a deeper understanding of precision medicine can empower nurses in supporting their patients as they are diagnosed with and treated for ovarian cancer.
The authors gratefully acknowledge Michele Galioto, DNP, RN, CNS, for her influence and contributions to the early design of this project.
About the Authors
Jaime Weimer, MSN, RN, AGCNS-BC, AOCNS®, is an oncology clinical specialist and Houtan Bozorghadad, BA, is a senior data analyst, both at the Oncology Nursing Society in Pittsburgh, PA; Keith Schoonover, MS, is vice president of research and Christine Carll, MA, is a research associate, both at the KJT Group in Rochester, NY; and Kirstin Repco, PMP, DASM, PMI-ACP, is a project manager at the Oncology Nursing Society. This research was supported, in part, by the Oncology Nursing Society through a sponsorship from AstraZeneca. Writing and editorial support were provided by Rebecca Hahn, MPH, of KJT Group, Inc., through funding from the Oncology Nursing Society. Schoonover and Carll are employees of KJT Group, Inc., which was contracted by the Oncology Nursing Society to conduct the study. Bozorghadad and Carll completed the data collection and provided statistical support. Weimer, Bozorghadad, Carll, and Repco provided the analysis and contributed to the manuscript preparation. All authors contributed to the conceptualization and design. Weimer can be reached at jweimer@ons.org, with copy to ONFEditor@ons.org. (Submitted November 2022. Accepted January 21, 2023.)
References
Abdelmeseh, V., Rogala, B., & Liauw, J. (2021, October 11). Overview of PARP inhibitors in the treatment of ovarian cancer. Pharmacy Times. https://www.pharmacytimes.com/view/overview-of-parp-inhibitors-in-the-t…
American Cancer Society. (2023a). Key statistics for breast cancer. https://www.cancer.org/cancer/breast-cancer/about/how-common-is-breast-…’s%20estimates,will%20die%20from%20breast%20cancer
American Cancer Society. (2023b). Key statistics for lung cancer. https://www.cancer.org/cancer/lung-cancer/about/key-statistics.html#:~:…’s%20estimates,men%20and%2061%2C360%20in%20women
American Cancer Society. (2023c). Key statistics for ovarian cancer. https://www.cancer.org/cancer/ovarian-cancer/about/key-statistics.html
Anand, P., Kunnumakkara, A.B., Sundaram, C., Harikumar, K.B., Tharakan, S.T., Lai, O.S., . . . Aggarwal, B.B. (2008). Cancer is a preventable disease that requires major lifestyle changes. Pharmaceutical Research, 25(9), 2097–2116. https://doi.org/10.1007/s11095-008-9661-9
AstraZeneca. (2020). Lynparza® (olaparib) [Package insert]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/208558s014lbl…
Basu, P., Mukhopadhyay, A., & Konishi, I. (2018). Targeted therapy for gynecologic cancers: Toward the era of precision medicine. International Journal of Gynaecology and Obstetrics, 143(Suppl. 2), 131–136. https://doi.org/10.1002/ijgo.12620
Chakravarty, D., Johnson, A., Sklar, J., Lindeman, N.I., Moore, K., Ganesan, S., . . . Meric-Bernstam, F. (2022). Somatic genomic testing in patients with metastatic or advanced cancer: ASCO provisional clinical opinion. Journal of Clinical Oncology, 40(11), 1231–1258. https://doi.org/10.1200/JCO.21.02767
Clovis Oncology. (2022). Rubraca® (rucaparib) [Package insert]. https://clovisoncology.com/pdfs/RubracaUSPI.pdf
Diab, Y., & Muallem, M.Z. (2017). Targeted therapy in ovarian cancer. A comprehensive systematic review of literature. Anticancer Research, 37(6), 2809–2815. https://doi.org/10.21873/anticanres.11631
Endo, Y., Watanabe, T., Saito, M., Saito, K., Suzuki, R., Sano, H., . . . Fujimori, K. (2022). A rare case of recurrent ovarian cancer with TPM3-NTRK1 gene rearrangement: A case report. Molecular and Clinical Oncology, 16(4), 90. https://doi.org/10.3892/mco.2022.2523
Evans, T., & Matulonis, U. (2017). PARP inhibitors in ovarian cancer: Evidence, experience and clinical potential. Therapeutic Advances in Medical Oncology, 9(4), 253–267. https://doi.org/10.1177/1758834016687254
Falzone, L., Salomone, S., & Libra, M. (2018). Evolution of cancer pharmacological treatments at the turn of the third millennium. Frontiers in Pharmacology, 9, 1300. https://doi.org/10.3389/fphar.2018.01300
Gadducci, A., Cosio, S., Carpi, A., Nicolini, A., & Genazzani, A.R. (2004). Serum tumor markers in the management of ovarian, endometrial and cervical cancer. Biomedicine and Pharmacotherapy, 58(1), 24–38. https://doi.org/10.1016/j.biopha.2003.11.003
GlaxoSmithKline. (2020). Zejula (niraparib) [Package insert]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/208447s015s01…
Guan, L.-Y., & Lu, Y. (2018). New developments in molecular targeted therapy of ovarian cancer. Discovery Medicine, 26(144), 219–229.
Hall, M., Gourley, C., McNeish, I., Ledermann, J., Gore, M., Jayson, G., . . . Kaye, S. (2013). Targeted anti-vascular therapies for ovarian cancer: Current evidence. British Journal of Cancer, 108(2), 250–258. https://doi.org/10.1038/bjc.2012.541
Hanes, E. (2020). Why cancer treatment is moving away from chemotherapy. Healthgrades. https://www.healthgrades.com/right-care/cancer/why-cancer-treatment-is-…
Hollasch, M. (2022). Nurses play key role in facilitating maintenance therapy with PARP inhibitors in ovarian cancer. Oncology Nursing News. https://www.oncnursingnews.com/view/nurses-play-key-role-in-facilitatin…
Jiang, T., Wang, G., Liu, Y., Feng, L., Wang, M., Liu, J., . . . Ouyang, L. (2021). Development of small-molecule tropomyosin receptor kinase (TRK) inhibitors for NTRK fusion cancers. Acta Pharmaceutica Sinica. B, 11(2), 355–372. https://doi.org/https://doi.org/10.1016/j.apsb.2020.05.004
Juretzka, M.M., Barakat, R.R., Chi, D.S., Iasonos, A., Dupont, J., Abu-Rustum, N.R., . . . Sabbatini, P. (2007). CA125 level as a predictor of progression-free survival and overall survival in ovarian cancer patients with surgically defined disease status prior to the initiation of intraperitoneal consolidation therapy. Gynecologic Oncology, 104(1), 176–180. https://doi.org/10.1016/j.ygyno.2006.07.027
Kandalaft, L.E., Odunsi, K., & Coukos, G. (2020). Immune therapy opportunities in ovarian cancer. American Society of Clinical Oncology Educational Book, 40, 1–13. https://doi.org/10.1200/EDBK_280539
Konstantinopoulos, P.A., Lacchetti, C., & Annunziata, C.M. (2020). Germline and somatic tumor testing in epithelial ovarian cancer: ASCO guideline summary. JCO Oncology Practice, 16(8), e835–e838. https://doi.org/10.1200/JOP.19.00773
Konstantinopoulos, P.A., Lheureux, S., & Moore, K.N. (2020). PARP inhibitors for ovarian cancer: Current indications, future combinations, and novel assets in development to target DNA damage repair. American Society of Clinical Oncology Educational Book, 40, 1–16. https://doi.org/10.1200/EDBK_288015
Lassen, U.N., Makaroff, L.E., Stenzinger, A., Italiano, A., Vassal, G., Garcia-Foncillas, J., & Avouac, B. (2021). Precision oncology: A clinical and patient perspective. Future Oncology, 17(30), 3995–4009. https://doi.org/10.2217/fon-2021-0688
Lim, H.J., & Ledger, W. (2016). Targeted therapy in ovarian cancer. Women’s Health, 12(3), 363–378. https://doi.org/10.2217/whe.16.4
Mateo, J., Steuten, L., Aftimos, P., André, F., Davies, M., Garralda, E., . . . Voest, E. (2022). Delivering precision oncology to patients with cancer. Nature Medicine, 28(4), 658–665. https://doi.org/10.1038/s41591-022-01717-2
Miller, J.A. (2022). Changing the treatment of cancer: Breakthroughs in the field of genomics are revolutionizing prevention, diagnosis and care. University of Michigan. https://healthblog.uofmhealth.org/cancer-care/changing-treatment-of-can…
Nag, S., Aggarwal, S., Rauthan, A., & Warrier, N. (2022). Maintenance therapy for newly diagnosed epithelial ovarian cancer—A review. Journal of Ovarian Research, 15(1), 88. https://doi.org/10.1186/s13048-022-01020-1
National Cancer Institute. (n.d.). NCI dictionary of cancer terms: Precision medicine. U.S. Department of Health and Human Services, National Institutes of Health. https://www.cancer.gov/publications/dictionaries/cancer-terms/def/preci…
National Comprehensive Cancer Network. (2022a). NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Ovarian cancer [v.4.2022]. https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf
National Comprehensive Cancer Network. (2022b). NCCN Guidelines for Patients®: Ovarian cancer. https://www.nccn.org/patients/guidelines/content/PDF/ovarian-patient.pdf
Nero, C., Ciccarone, F., Pietragalla, A., Duranti, S., Daniele, G., Salutari, V., . . . Lorusso, D. (2021). Ovarian cancer treatments strategy: Focus on PARP inhibitors and immune check point inhibitors. Cancers, 13(6), 1298. https://doi.org/10.3390/cancers13061298
Nevidjon, B.M. (2018). Oncology nurses: Innovating precision care in a changing treatment environment. Asia-Pacific Journal of Oncology Nursing, 5(2), 131–133. https://doi.org/10.4103/apjon.apjon_1_18
O’Malley, D.M., Arend, R.C., Alam, N., Ozgoren, O., McLaurin, K.K., Long, G.H., & Banerjee, S.N. (2022). Real-world use, tolerability, and dose modifications of PARP inhibitors in ovarian cancer. Journal of Clinical Oncology, 40(Suppl. 16), 5552. https://doi.org/10.1200/JCO.2022.40.16_suppl.5552
Oncology Nursing Society. (n.d.). Role of the oncology nurse navigator throughout the cancer trajectory [Position statement]. https://www.ons.org/make-difference/advocacy-and-policy/position-statem…
Oncology Nursing Society. (2021). Clinical update: A focus group on immuno-oncology, navigation, precision oncology, and immune-related adverse events. https://www.ons.org/articles/clinical-update-focus-group-immuno-oncolog…
Oncology Nursing Society. (2022). Clinical update: Focus on biomarker testing and new therapies in ovarian cancer. https://www.ons.org/clinical-practice-resources/clinical-update-parp-in…
O’Sullivan, C.K., Bowles, K.H., Jeon, S., Ercolano, E., & McCorkle, R. (2011). Psychological distress during ovarian cancer treatment: Improving quality by examining patient problems and advanced practice nursing interventions. Nursing Research and Practice, 2011, 351642. https://doi.org/10.1155/2011/351642
Pothuri, B., O’Cearbhaill, R., Eskander, R., & Armstrong, D. (2020). Frontline PARP inhibitor maintenance therapy in ovarian cancer: A society of gynecologic oncology practice statement. Gynecologic Oncology, 159(1), 8–12. https://doi.org/10.1016/j.ygyno.2020.07.097
Reed, L.M., & Rua, K. (2020). Defining the role of the oncology nurse navigator. Journal of Oncology Navigation and Survivorship, 11(3). https://www.jons-online.com/issues/2020/march-2020-vol-11-no-3/2842-def…
Ruddy, K.J., Risendal, B.C., Garber, J.E., & Partridge, A.H. (2016). Cancer survivorship care: An opportunity to revisit cancer genetics. Journal of Clinical Oncology, 34(6), 539–541. https://doi.org/10.1200/jco.2015.63.5375
Santillan, A., Garg, R., Zahurak, M.L., Gardner, G.J., Giuntoli, R.L., 2nd, Armstrong, D.K., & Bristow, R.E. (2005). Risk of epithelial ovarian cancer recurrence in patients with rising serum CA-125 levels within the normal range. Journal of Clinical Oncology, 23(36), 9338–9343. https://doi.org/10.1200/jco.2005.02.2582
Senft, D., Leiserson, M.D.M., Ruppin, E., & Ronai, Z.A. (2017). Precision oncology: The road ahead. Trends in Molecular Medicine, 23(10), 874–898. https://doi.org/10.1016/j.molmed.2017.08.003
Shahid, K., Khalife, M., Dabney, R., & Phan, A.T. (2019). Immunotherapy and targeted therapy—The new roadmap in cancer treatment. Annals of Translational Medicine, 7(20), 595. https://doi.org/10.21037/atm.2019.05.58
Slade, D. (2020). PARP and PARG inhibitors in cancer treatment. Genes and Development, 34(5-6), 360–394. https://doi.org/10.1101/gad.334516.119
Temkin, S.M., Smeltzer, M.P., Dawkins, M.D., Boehmer, L.M., Senter, L., Black, D.R., . . . Thaker, P.H. (2022). Improving the quality of care for patients with advanced epithelial ovarian cancer: Program components, implementation barriers, and recommendations. Cancer, 128(4), 654–664. https://doi.org/10.1002/cncr.34023
Tsaousis, G.N., Papadopoulou, E., Apessos, A., Agiannitopoulos, K., Pepe, G., Kampouri, S., . . . Nasioulas, G. (2019). Analysis of hereditary cancer syndromes by using a panel of genes: Novel and multiple pathogenic mutations. BMC Cancer, 19(1), 535. https://doi.org/10.1186/s12885-019-5756-4
Wang, H., Wu, M., Liu, H., Zhou, H., Zhao, Y., Geng, Y., . . . Du, X. (2021). Comparison of the efficacy and safety of PARP inhibitors as a monotherapy for platinum-sensitive recurrent ovarian cancer: A network meta-analysis. Frontiers in Oncology, 11, 785102. https://doi.org/10.3389/fonc.2021.785102
Wang, Q., Peng, H., Qi, X., Wu, M., & Zhao, X. (2020). Targeted therapies in gynecological cancers: A comprehensive review of clinical evidence. Signal Transduction and Targeted Therapy, 5(1), 137. https://doi.org/10.1038/s41392-020-0199-6
Zhang, Y., Yi, S., Trace, C.B., & Williams-Brown, M.Y. (2022). Understanding the information needs of patients with ovarian cancer regarding genetic testing to inform intervention design: Interview study. JMIR Cancer, 8(1), e31263.


